General Properties of Nucleus:
(i) Nuclear Size:
From Rutherford’s work, the mean radius of nucleus is 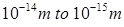 and the radius of the atom is
and the radius of the atom is 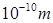 . Thus the radius of the nucleus is 10,000 times smaller than the radius of the atom.
. Thus the radius of the nucleus is 10,000 times smaller than the radius of the atom.
The empirical formula for the nuclear radius is,
Here, A is the mass number,  is a constant.
is a constant.
From this formula the radius of the Carbon ( ) nucleus is.
) nucleus is.
(ii) Nuclear Mass:
If there are Z protons and N neutrons then the assumed mass is,
Where,  is the mass of each proton and
is the mass of each proton and  is mass of each neutron. But,
is mass of each neutron. But,
Difference of the masses,
Δm is called the mass defect.
(iii) Nuclear Density:
Here, nuclear mass =  , where A is the mass number and
, where A is the mass number and 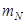 is the mass of the nucleon =
is the mass of the nucleon = 









