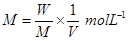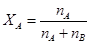Molarity (M):
Molarity is defined as the number of moles of solute per litter of solution. That is,
Here,
W = weight of solute.
M = molecular mass of solute
V = volume of solution
Molality (m):
It is defined as the number of moles of solute per kg of solvent. That is,
Here,
b = weight of solvent
Normality (N):
It is defined as the number of gram equivalents of the solute dissolved per litter of the solution at a specific temperature.
Mole-fraction (x):
Mole fraction is the ratio of the number of moles of solute and the total number of moles of solute and solvent. The mole fraction of any constituent of a solution is defined as the number of moles of that constituent per mole of the solution. If the solution contained moles 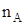 of A and
of A and 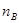 moles of B, then
moles of B, then