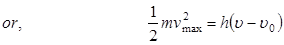Einstein’s Photoelectric Equation :
According to Einstein, light of frequency  consists of a shower of photons, is of energy
consists of a shower of photons, is of energy  . When a photon of light of frequency
. When a photon of light of frequency  is incident on a metal, a part of the energy acquired by the electron is used to pull out the electron from the surface of the metal and the rest of it is utilized in imparting K.E. to the emitted electron.
is incident on a metal, a part of the energy acquired by the electron is used to pull out the electron from the surface of the metal and the rest of it is utilized in imparting K.E. to the emitted electron.
 consists of a shower of photons, is of energy
consists of a shower of photons, is of energy  . When a photon of light of frequency
. When a photon of light of frequency  is incident on a metal, a part of the energy acquired by the electron is used to pull out the electron from the surface of the metal and the rest of it is utilized in imparting K.E. to the emitted electron.
is incident on a metal, a part of the energy acquired by the electron is used to pull out the electron from the surface of the metal and the rest of it is utilized in imparting K.E. to the emitted electron.